Investigate the amount of hydrochloric acid neutralised by selected indigestion tablets, as one measure of their effectiveness
The experiment is part of the Nuffield practical collection, developed by the Nuffield Foundation and the Royal Society of Chemistry. Delve into a wide range of chemical concepts and processes with this collection of over 200 step-by-step practicals.
Use with our How to teach titration infographic poster from Education in Chemistry. The poster and fact sheet illustrate the apparatus and techniques needed to carry out a successful titration experiment.
Learning objectives
- Follow a method and carry out a titration to determine which brand of indigestion tablet contains the most active ingredient.
- Explain the reasons behind particular steps in the titration procedure.
- Explain how procedural errors can contribute towards inaccurate titres.
Introduction
Titration is a long-established analytical technique. At a basic level it uses a reaction of a volume of a solution of known concentration to find out the concentration of an unknown solution. There are many different types of titration; this practical focuses on an acid-base reaction.
Indigestion tablets (antacids) contain a mixture of substances which can neutralise stomach acid. These include carbonates of group 2 metals (e.g. calcium carbonate) and group 2 hydroxides (e.g. magnesium hydroxide). In this experiment, learners analyse different brands of indigestion tablet and use the average titres of the titration to determine which tablet contains the most active ingredient.
The focus is on the experiment itself rather than the generation of results which can be used in a classic titration calculation. This makes the experiment ideal to use as learners make the transition from 14-16 chemistry.
The experiment to test a single tablet should take no more than 25 minutes and learners should get quicker as they do repeats. Allocate different groups one ‘standardisation’ brand of tablet to test and one other brand, so that the class can compare a number of different brands in a one-hour lesson.
Scaffolding
Learners in this age range are on their journey towards independence in carrying out practical procedures. Scaffold the investigation to support learners with limited practical experience using the following suggestions:
- Ask learners to read the method and look at the poster or watch a video on how to use a burette prior to the practical session.
- Demonstrate the correct method for using a burette either in this lesson or a prior lesson.
- Provide samples with the correct colour match for the end point with methyl orange.
For more challenge, extend this practical as a back-titration technique.
Equipment
Per person:
- Eye protection: safety glasses to EN166 F or (EN) ISO 16321 C.
Per group:
- Burette, 30 cm3 or 50 cm3 capacity
- Conical flask, 100 cm3
- Beaker, 100 cm3
- Pestle and mortar
- Stirring rod
- Spatula
- Filter funnel, small (about 35 mm diameter)
- White tile (optional)
- Burette stand and clamp
Chemicals
- Dilute hydrochloric acid, concentration equal to or below 0.4 mol dm-3, 100 cm3
- Two or three different indigestion tablets, ideally the chalky type. IT is sensible to select brands for which a comparison is straightforward, with active ingredients restricted to carbonates, bicarbonates and/or hydroxides, avoiding those containing other active ingredients.
- Original packets from which the tablets are taken, together with price information for each packet
- Methyl orange indicator solution (or alternative) DANGER: Harmful if swallowed. May cause damage to organs. Highly flammable liquid and vapour.
- Dionised or distilled water, about 100 cm3
Technician notes
Separate technician notes, including information about the preparation and disposal and links to relevant CLEAPSS Hazcards and recipe sheets, are available to download as MS Word or PDF.
Read our standard health and safety guidance and carry out a risk assessment before running any live practical.
Titration procedure
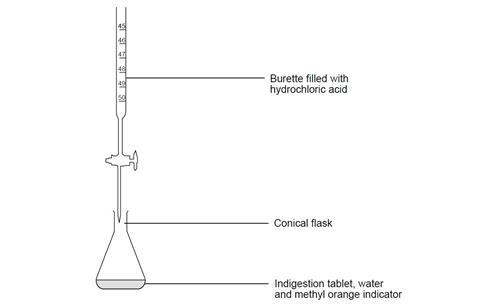
Wear safety glasses.
- Check which indigestion tablets you have been assigned to analyse by your teacher. Read the packet and note down which active ingredients are present.
- Crush a tablet using a pestle and mortar and carefully transfer it to a conical flask, using a spatula to ensure complete transfer as far as possible. Rinse any remaining fragments into the flask with a few cm3 of deionised water.
- Add about 25 cm3 of deionised water to the flask, followed by three drops of methyl orange indicator. DANGER: Harmful if swallowed. May cause damage to organs. Highly flammable liquid and vapour.
- Using a small funnel, pour a few cm3 of the dilute hydrochloric acid provided into the burette, with the tap open and a beaker under the open tap. Once the tip of the burette is full of solution, close the tap and add more of the solution up to the zero mark. (Do not reuse the acid in the beaker – this should be rinsed down the sink.) Remove the funnel.
- Add acid from the burette into the flask, 1–2 cm3 at a time, while slowly swirling the flask. Continue to add the acid until you begin to see a red colour in the flask that quickly returns to yellow-orange.
- When this begins to take longer to happen, add a smaller quantity of acid at a time – eg 0.5 cm3 – until you reach a point where the red colour remains after one minute.
- Record the volume of acid used.
- Rinse the flask with water, and repeat the experiment with the same indigestion tablet until you have concordant results (within 0.10 cm3 of each other) or have done the titration 3 times. Refill the burette, if necessary.
Teaching notes
Titrating a powdered tablet containing insoluble ingredients such as calcium carbonate, magnesium carbonate and magnesium hydroxide is slow, as you need to allow for the solid to react with the acid. If the tablets have been pretested for their expected titre values, learners can be instructed to add acid from the burette rapidly to a point 5 cm3 below the lowest expected value for the brands being tested – this should save time.
Student questions
You will find follow-up questions to this practical investigation in the student worksheet, available in MS Word and PDF.
The questions cover aspects of both the titration procedure and the analysis of results. The experiment is designed to raise ‘fair test’ principles for discussion, and students are expected to comment with rational arguments on the validity of the comparisons they make. In particular, and if possible without prompting, they ought to read the instructions on each packet concerning the recommended dosage. Many comparisons are likely to be ‘grey’ rather than ‘black and white’. This could lead to suggestions for further investigations for improving comparisons, but it is unlikely that these will be feasible at school.
Answers to the questions are in the Teacher notes, also available in MS Word and PDF.
More resources
- Use the Mastering titration apparatus poster, fact sheet and resource to get your learners familiar with the equipment before they do a titration.
- Find ideas for successfully teaching titration, how to connect to theory and common misconceptions.
- From modelling to scaffolding, try these five approaches to help students master titration.
- Write balanced equations and calculate reacting masses and moles to find the limiting reagent with this acid-base back titration for learners aged 16–18 years.
- Learn how to prepare a standard solution, calculate the concentration of an unknown acid or moles of a known solid, and understand the different types of titration with these practical videos for 16–18 year-old learners.
- Bioanalytical scientist, Claire, analyses drug concentrations in biological samples to ensure new medicines are robust and reliable before being released onto the consumer market.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. The resource was updated in 2024 with new learner-facing questions written by Kristy Turner and new student, teacher and technician downloads added to accompany the How to teach titration infographic poster in Education in Chemistry .
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry
























10 readers' comments